WMS 2021 Day 3: Opening Lecture and Debates

WMS 2021’s agenda on Wednesday, September 22nd was not so much about specific diseases, but rather major and timely research themes across all of biology. The day started with a keynote lecture on the promise and limitations of artificial intelligence (AI) in drug discovery from Brendan Frey, Founder and CEO of the company Deep Genomics. His talk was an excellent overview of how AI and deep learning is used in biology and medicine, the types of questions they’re good at addressing, and where the field could still evolve for maximum impact.
The rest of the day focused on a series of three debates where the pros and cons of different experimental and clinical approaches were discussed in detail. One debate focused on the use of small animal models (fish, mice) versus large animal models (dogs, pigs, primates) in translational research. Another discussed using devices (like activity monitors) versus having humans evaluate performance in clinical trials. The third debate discussed the impacts of newborn screening versus pre-conception carrier testing. For each of these topics, the conversations weighed the myriad of factors that come into play—cost, feasibility, ethics, quality of data, relevance of results, etc. Interestingly, the take-away for all of them was largely the same—that we need both options because different questions are better served by one or the other approaches. The key is always to think smartly about what you are trying to achieve, and to pair the approach that best addresses the question at hand.
Poster highlight: pre-existing AAV antibodies
Another major component of the WMS conference are the poster presentations. As usual, there are a lot of posters relevant to Duchenne – at least 100 of the conference’s 391 posters total. While we won’t be able to summarize them all, today we’re highlighting a poster from Sarepta.
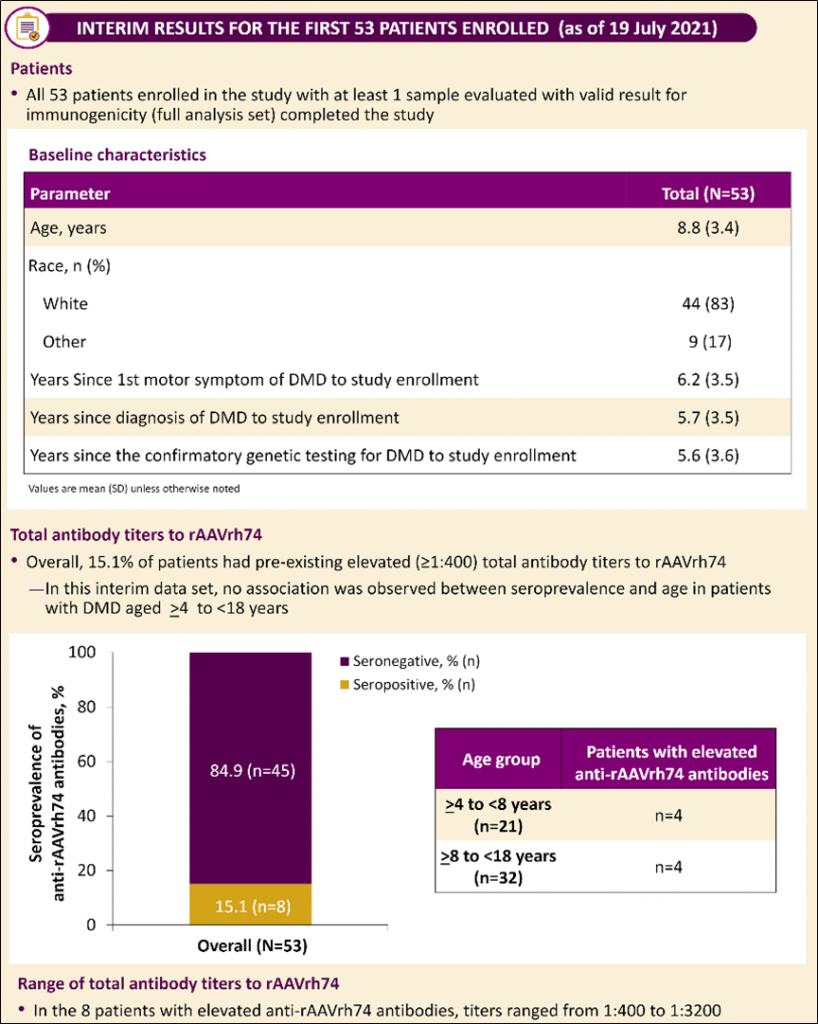
Sarepta reported an interesting interim analysis evaluating total binding antibodies (Ab’s) against the rAAVrh74 virus in a small number of Duchenne muscular dystrophy patients participating in their gene therapy clinical trial.
Total anti-AAV antibodies include both neutralizing Ab’s (which will directly prevent transduction of the vector) and non-neutralizing Ab’s, with values up to 1:400 being used to define low or “not elevated” levels. This cutoff was based on published studies that have shown Ab titers of 1:800 could produce a reduction or loss of transgene expression.
Of the first 53 Duchenne patients (4 – 18 years old) examined, 8 (15.1%) had pre-existing elevated total Ab titers to rAAVrh74 (titers ranges from 1:400 to 1:3200) while 45 patients (84.5%) were considered seronegative. The interim analysis did not to show an association between seroprevalence and age in this patient population.
These early results, albeit from a very small sample of patients, support the applicability of rAAVrh74 in gene therapy applications.